(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-What is the sign of the enthalpy of reaction for the reaction P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?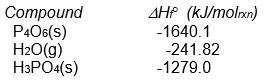
A) positive
B) negative
C) impossible to determine from the data
Correct Answer:
Verified
Q51: (Note that some of these
Q52: (Note that some of these
Q53: (Note that some of these
Q54: (Note that some of these
Q55: (Note that some of these are
Q57: (Note that some of these are
Q58: (Note that some of these
Q59: (Note that some of these
Q60: The reaction below shows two isomers of
Q61: Retailers purchase gasoline by weight so they
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents