The outermost or highest energy electron in element 105 could be characterized by which of the following sets of n, l, ml and ms quantum numbers?
A) 7, 3, -3, - 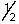
B) 6, 3, -1, - 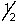
C) 6, 2, 0, 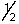
D) 5, 3, -1, - 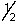
E) 5, 2, 0, 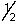
Correct Answer:
Verified
Q72: A single atom of element 114 was
Q73: Theoreticians predict that the element with atomic
Q74: In period 6 of the periodic table,
Q75: A possible set of quantum numbers for
Q76: A possible set of quantum numbers for
Q78: Which of the following describes a possible
Q79: What is the lowest energy electronic configuration
Q80: What is the value of x in
Q81: What is the correct electron configuration for
Q82: Which of the following would have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents