Which of the following describes a possible set of quantum numbers for the last electron added to form an aluminum atom when atomic orbitals are filled?
A) 1, 0, 0, + 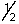
B) 2, 0, 0, + 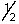
C) 2, l, 1, + 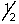
D) 3, 0, 0, + 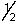
E) 3, 1, 1, + 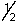
Correct Answer:
Verified
Q73: Theoreticians predict that the element with atomic
Q74: In period 6 of the periodic table,
Q75: A possible set of quantum numbers for
Q76: A possible set of quantum numbers for
Q77: The outermost or highest energy electron in
Q79: What is the lowest energy electronic configuration
Q80: What is the value of x in
Q81: What is the correct electron configuration for
Q82: Which of the following would have the
Q83: What is the correct electron configuration for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents