Multiple Choice
On April 19, 1995 a bomb exploded, killing 168 people, wounding hundreds of others and destroying the Alfred P. Murrah Federal Building in Oklahoma City. The explosive mixture used in this incident contained ammonium nitrate, NH4NO3, which is commonly used as a fertilizer. What is the molarity of concentrated nitric acid, HNO3(aq) , if it takes 5.63 mL of the acid to consume 15.00 mL of 6.00 M NH3(aq) ?
NH3(aq) + HNO3(aq) 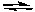 NH4NO3(aq)
NH4NO3(aq)
A) less than 1 M
B) between 1 and 5 M
C) between 5 and 10 M
D) between 10 and 15 M
E) more than 15 M
Correct Answer:
Verified
Related Questions

