Two processes are shown on the p-V graph in Figure 12-1; one is an adiabat and the other is an isotherm. Is the process represented by the upper curve an adiabat or an isotherm?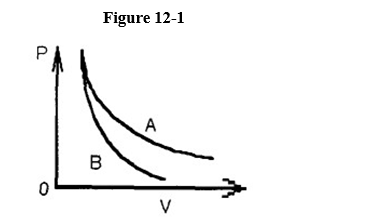
Correct Answer:
Verified
Q2: Why don't we use "efficiency" for rating
Q3: Is it possible for heat to travel
Q4: We say that energy cannot be created
Q5: Both the refrigerator and the heat pump
Q6: As a system loses the ability to
Q7: Entropy is a measure of order. The
Q8: Entropy of the universe is increasing.
Q9: A Carnot cycle requires an ideal gas
Q10: Change in internal energy is
A) Qout/Win.
B) 1
Q11: The First Law of Thermodynamics is equivalent
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents