Reaction conditions for a hypothetical reaction, A + B C, are given below.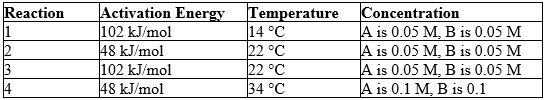
Predict which reaction should occur at the slowest rate. (M = molarity)
A) 1
B) 2
C) 3
D) 4
Correct Answer:
Verified
Q8: Reaction A releases 24 kJ/mole and has
Q9: Reaction conditions for a hypothetical reaction,
Q10: Which response includes all the factors that
Q11: Increasing the temperature of a chemical reaction
Q12: At equilibrium, the equilibrium constant for
Q14: Chemical equilibrium is reached in a system
Q15: A mixture of 1.40 moles of
Q16: A chemical equilbrium expression depends on the
Q17: Given the following reaction, the equilibrium
Q18: Which of the following is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents