The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M) 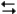 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. Do you expect the silver electrode to gain mass or lose mass as the reaction approaches equilibrium?
A) Gain mass
B) Lose mass
C) Neither, solids do not appear in the equilibrium constant expression
D) Not enough information is given to answer the question
E) None of the above
Correct Answer:
Verified
Q2: An electrochemical system in which a 1.5
Q3: An electrochemical system in which a 1.5
Q4: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q5: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q6: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q7: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q9: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q10: The electrochemical reaction below at 25°C written
Q11: The electrochemical reaction below at 25°C written
Q12: In the following equation ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents