The electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M) 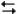 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
-Refer to Exhibit 17-3. What will be the concentration of Fe3+ when the reaction reaches equilibrium?
A) 0.183 M
B) 0.217 M
C) 0.112 M
D) 0.290 M
E) None of the above
Correct Answer:
Verified
Q2: An electrochemical system in which a 1.5
Q3: An electrochemical system in which a 1.5
Q4: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q5: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q6: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q7: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q8: The electrochemical reaction below at 25°C.
Ag+(0.275 M)
Q10: The electrochemical reaction below at 25°C written
Q11: The electrochemical reaction below at 25°C written
Q12: In the following equation ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents