What is the correct order of reagents needed for the following reaction? 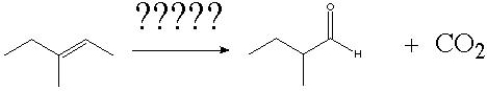
A) 1) HBr/CCl4 2) Sodium Methoxide/Methanol 3) KMnO4, OH-, H2O, heat
B) 1) HBr/CCl4 2) Sodium Methoxide/Methanol 3) O3, CH2Cl2, - 78°C 4)
Zn/AcOH
C) 1) HBr/H2O2 2) Sodium Methoxide/Methanol 3) O3,CH2Cl2, - 78°C 4) Zn/AcOH
D) 1) HBr/H2O2 2) Potassium tert-butoxide 3) KMnO4, OH-, H2O, heat
E) 1) HBr/H2O22) Potassium tert-butoxide 3) O3, CH2Cl2, -78°C 4) Zn/AcOH
Correct Answer:
Verified
Q1: The Oxymercuration-Demercuration reaction produces a _ addition.
A)
Q2: The Hydroboration-Oxidation reaction produces a _ addition.
A)
Q3: The addition of Br2 in CCl4 to
Q4: What is the product of the following
Q5: _ reactions form products where the reagent
Q6: Which of the following reagents produces Markovnikov
Q7: What is the product of the following
Q8: What is the product of the following
Q9: What is the product of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents