Draw Lewis structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. Determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. Explain what makes the other molecules nonsensical, considering the number of bonds each type of atom can make.
a.
c.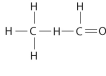
b.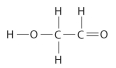
d. 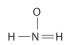
Correct Answer:
Verified
Q73: Refer to the following figure to answer
Q74: Which of the following describes any reaction
Q75: A group of molecular biologists is trying
Q75: Which of the following best describes chemical
Q76: Refer to the following figure to answer
Q77: Refer to the following figure to answer
Q79: Compared with 31P, the radioactive isotope ³²P
Q80: Atoms can be represented by simply listing
Q81: Which of the following statements correctly describes
Q82: What coefficients must be placed in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents