For the hydrolysis of ATP to ADP + 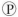 ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and
ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and  ᵢ under cellular conditions?
ᵢ under cellular conditions?
A) It is +7.3 kcal/mol.
B) It is less than +7.3 kcal/mol.
C) It is about +13 kcal/mol.
D) It is greater than +13 kcal/mol.
E) The information given is insufficient to deduce the free energy change.
Correct Answer:
Verified
Q23: According to the induced fit hypothesis of
Q25: Mutations that result in single amino acid
Q28: Reactants capable of interacting to form products
Q29: The active site of an enzyme is
Q31: A number of systems for pumping ions
Q31: Which of the following is most similar
Q34: During a laboratory experiment, you discover that
Q36: When chemical, transport, or mechanical work is
Q39: What is the difference (if any)between the
Q40: Which of the following statements is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents