An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
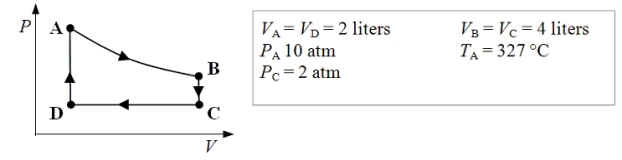
-What is the temperature of the gas when it is in state C?
A) -33 °C
B) 130 °C
C) 327 °C
D) 817 °C
E) 1500 °C
Correct Answer:
Verified
Q69: A heat engine operates between a hot
Q70: A heat engine operates between a hot
Q71: Which one of the following processes represents
Q72: An ideal monatomic gas expands isothermally from
Q73: An ideal monatomic gas expands isothermally from
Q74: A 1.00-kg sample of steam at 100.0
Q75: A heat engine operates between a hot
Q76: A container holding 1.2 kg of water
Q77: An ideal monatomic gas expands isothermally from
Q79: In an isothermal and reversible process,945 J
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents