An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
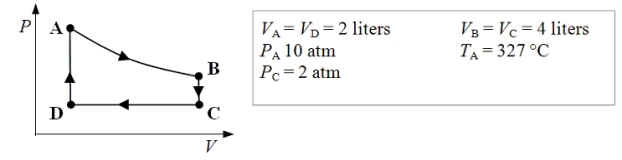
-What is the net amount of work done after one complete cycle?
A) zero joules
B) 20 J
C) 40 J
D) 1000 J
E) 1340 J
Correct Answer:
Verified
Q68: A container holding 1.2 kg of water
Q69: A heat engine operates between a hot
Q70: A heat engine operates between a hot
Q71: Which one of the following processes represents
Q72: An ideal monatomic gas expands isothermally from
Q74: A 1.00-kg sample of steam at 100.0
Q75: A heat engine operates between a hot
Q76: A container holding 1.2 kg of water
Q77: An ideal monatomic gas expands isothermally from
Q78: An ideal monatomic gas expands isothermally from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents