When 50.0 mL of 0.400 mol L-1 Ca(NO3) 2 is added to 50.0 mL of 0.800 mol L-1 NaF, CaF2 precipitates, as shown in the net ionic equation below. The initial temperature of both solutions is 23.0 °C. Assuming that the reaction goes to completion, and that the resulting solution has a mass of 100.00 g and a specific heat of 4.18 J g-1°C-1, calculate the final temperature of the solution. Ca2+(aq) + 2F-(aq) → CaF2(s) 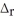 H = -11.5 kJ
H = -11.5 kJ
A) 22.45 °C
B) 23.55 °C
C) 24.10 °C
D) 24.65 °C
Correct Answer:
Verified
Q111: For a particular process that is carried
Q116: 8.000 moles of H2(g)reacts with 4.000 mol
Q126: Hydrogen peroxide decomposes to water and oxygen
Q127: At 1.0133 bar, the heat of sublimation
Q128: The specific heat capacity of methane gas
Q129: The specific heat of copper is 0.385
Q130: How much heat is absorbed when 50.00
Q133: Match the following.
-energy associated with the motion
Q134: What is the enthalpy change (in kJ)of
Q136: The combustion of titanium with oxygen produces
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents