Write a balanced reaction for which the following rate relationships are true. Rate = - 
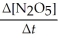 =
= 
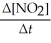 =
= 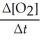
A) 2N2O5 → 4NO2 + O2
B) 4NO2 + O2 → 2N2O5
C) 2N2O5 → NO2 + 4O2
D)  NO2 + O2 →
NO2 + O2 →  N2O5
N2O5
E)  N2O5 →
N2O5 →  NO2 + O2
NO2 + O2
Correct Answer:
Verified
Q4: Given the following balanced equation, determine the
Q8: Without performing complex calculations, determine the order
Q10: Without performing complex calculations, determine the order
Q11: Given the following balanced equation, determine the
Q12: Given the following balanced equation, determine the
Q14: Write a balanced reaction for which the
Q16: Given the following balanced equation, determine the
Q17: Without performing complex calculations, determine the order
Q18: Identify the methods used to monitor a
Q18: Given the following balanced equation, determine the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents