Without performing complex calculations, determine the order of a reaction that has the following initial rates for the specified starting concentration: 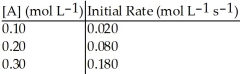
A) zero order
B) first order
C) second order
D) third order
E) impossible to determine
Correct Answer:
Verified
Q4: Given the following balanced equation, determine the
Q5: Given the following balanced equation, determine the
Q12: Given the following balanced equation, determine the
Q13: Write a balanced reaction for which the
Q14: Write a balanced reaction for which the
Q16: Given the following balanced equation, determine the
Q18: Given the following balanced equation, determine the
Q20: Given the following balanced equation, determine the
Q21: Without performing complex calculations, determine the order
Q22: What are the units of k in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents