Phosphorus trichloride and phosphorus pentachloride equilibrate in the presence of molecular chlorine according to the following reaction: 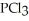 (g) +
(g) + 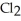 (g) →
(g) → 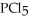 (g) An equilibrium mixture at 450 K contains
(g) An equilibrium mixture at 450 K contains 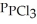 = 0.124 bar,
= 0.124 bar, 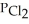 = 0.157 bar, and
= 0.157 bar, and 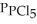 = 1.30 bar. What is the value of Kp at this temperature?
= 1.30 bar. What is the value of Kp at this temperature?
A) 66.8 bar-1
B) 1.50 × 10-2 bar-1
C) 2.53 × 10-2 bar-1
D) 1.02 bar-1
E) 4.63 bar-1
Correct Answer:
Verified
Q52: Consider the following reaction at equilibrium.What effect
Q72: Consider the following reaction at equilibrium.What effect
Q76: Consider the following reaction, equilibrium concentrations, and
Q79: The following reaction is exothermic. Which change
Q82: Consider the following reaction: NO(g) + SO3(g)
Q82: The decomposition of ammonia is 2NH3(g)→ N2(g)+
Q85: The Kp for the reaction below is
Q89: Consider the following reaction: CuS(s) + O2(g)
Q96: Consider the following reaction: COCl2(g) ⇌ CO(g)
Q104: Consider the following reaction at equilibrium. What
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents