How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 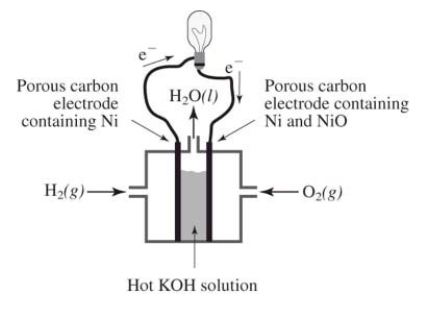 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
II) Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III) In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV) It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
A) I and II only
B) I,II,and III only
C) I,II,III,and IV
D) I and III only
Correct Answer:
Verified
Q8: Whenever a substance is oxidized,
A)it is called
Q9: Why doesn't water naturally become hydrogen and
Q10: In general,a modern hybrid vehicle is less
Q11: Reforming processes can store hydrogen fuel in
Q12: For the compound FeCl3,what is the oxidation
Q13: Chemical energy is converted directly into electrical
Q14: In a fuel cell,
A)there is oxidation,but no
Q15: Batteries are still preferred over supercapacitors for
Q16: Why is it so expensive to ship
Q17: Which of the following automotive energy storage
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents