Use the molecular orbital diagram shown to determine which of the following is MOST stable. 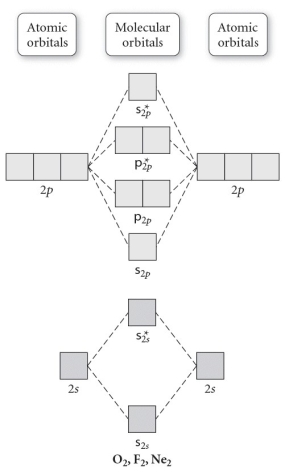
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer:
Verified
Q13: Give the electron geometry (eg),molecular geometry (mg),and
Q17: Use the molecular orbital diagram shown to
Q19: Give the electron geometry (eg),molecular geometry (mg),and
Q21: The hybrid orbital set used by the
Q22: Identify the number of electron groups around
Q23: Draw the molecular orbital diagram and determine
Q30: Which of the following statements is TRUE?
A)
Q32: Give the hybridization for the Br in
Q33: Give the hybridization for the Br in
Q35: Give the hybridization for the O in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents