Use the molecular orbital diagram shown to determine which of the following are paramagnetic. 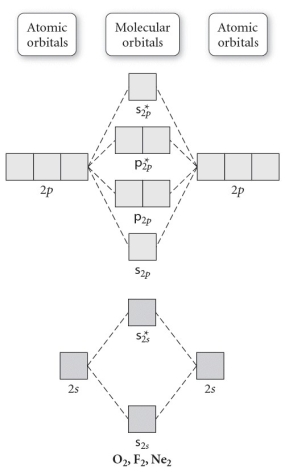
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above are paramagnetic.
Correct Answer:
Verified
Q13: Give the electron geometry (eg),molecular geometry (mg),and
Q18: Use the molecular orbital diagram shown to
Q19: Give the electron geometry (eg),molecular geometry (mg),and
Q21: How many of the following molecules have
Q21: The hybrid orbital set used by the
Q22: Identify the number of electron groups around
Q30: Which of the following statements is TRUE?
A)
Q32: Give the hybridization for the Br in
Q33: Give the hybridization for the Br in
Q35: Give the hybridization for the O in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents