Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2Mg(s) + 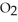 (g) → 2MgO(s)
(g) → 2MgO(s)
When 3.00 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
A) 3.00
B) 4.98
C) 0.123
D) 2.49
E) 10.0
Correct Answer:
Verified
Q69: If the percent yield for the following
Q70: 8.0 g of nitrogen is reacted with
Q72: 9.0 g of iron is reacted with
Q73: Calcium oxide reacts with water in a
Q74: Calcium oxide reacts with water in a
Q100: When 8.00 × 1022 molecules of ammonia
Q119: If the percent yield for the following
Q168: If 588 grams of FeS2 is allowed
Q232: Define a limiting reagent.
Q244: Balance the following equation:
_ C9H20 + _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents