Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 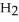 O(l) →
O(l) → 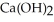 (s)
(s)
A 5.00-g sample of CaO is reacted with 4.83 g of 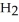 O.How many grams of water remain after the reaction is complete?
O.How many grams of water remain after the reaction is complete?
A) 0.00
B) 0.00991
C) 3.22
D) 1.04
E) 0.179
Correct Answer:
Verified
Q68: Lithium and nitrogen react in a combination
Q69: If the percent yield for the following
Q70: 8.0 g of nitrogen is reacted with
Q72: 9.0 g of iron is reacted with
Q74: Calcium oxide reacts with water in a
Q75: Magnesium burns in air with a dazzling
Q100: When 8.00 × 1022 molecules of ammonia
Q168: If 588 grams of FeS2 is allowed
Q232: Define a limiting reagent.
Q244: Balance the following equation:
_ C9H20 + _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents