Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + 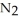 (g) →
(g) → 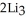 N(s)
N(s)
In a particular experiment,1.00-g samples of each reagent are reacted.The theoretical yield of lithium nitride is ________ g.
A) 1.01
B) 0.84
C) 5.0
D) 1.67
E) 2.50
Correct Answer:
Verified
Q69: If the percent yield for the following
Q70: 8.0 g of nitrogen is reacted with
Q72: 9.0 g of iron is reacted with
Q73: Calcium oxide reacts with water in a
Q83: When silver nitrate reacts with barium chloride,silver
Q109: When 14.0 g of calcium metal is
Q132: Which substance is the limiting reactant when
Q168: If 588 grams of FeS2 is allowed
Q237: Describe the greenhouse effect.
Q257: Balance the following equation:
_ C10H12 + _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents