The combustion of titanium with oxygen produces titanium dioxide: Ti(s) +  (g) →
(g) →  (s)
(s)
When 2.060 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00°C to 91.60°C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJ/K.The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ/mol.
A) 14.3
B) 19.6
C) -311
D) -0.154
E) -1.52 × 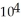
Correct Answer:
Verified
Q86: According to the following thermochemical equation,what mass
Q97: When 0.455 g of anthracene,C14H10,is combusted in
Q103: At 1 atm pressure,the heat of sublimation
Q104: Which of the following processes is exothermic?
A)
Q107: Identify what a bomb calorimeter measures.
A) measures
Q112: How much energy is evolved during the
Q113: For a particular process that is carried
Q121: When 1.50 mol of CH4(g)reacts with excess
Q131: How much heat is absorbed when 45.00
Q132: When 5.00 mol of benzene is vaporized
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents