In the presence of excess oxygen,methane gas burns in a constant-pressure system to yield carbon dioxide and water: 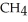 (g) +
(g) + 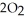 (g) →
(g) → 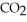 (g) +
(g) + 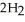 O(l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
O(l) ΔH = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
A) -94.6 kJ
B) 0.0306 kJ
C) -0.0106 kJ
D) 32.7 kJ
E) -9.46 × 104 kJ
Correct Answer:
Verified
Q123: When 1.50 g of Ba(s)is added to
Q124: Describe the energy changes that occur when
Q124: Choose the reaction that illustrates ΔH°f for
Q127: Identify what a coffee cup calorimeter measures.
A)
Q129: Identify an element that is NOT in
Q131: Where does the energy absorbed during an
Q132: Choose the reaction that illustrates ΔH°f for
Q133: If a block of gold requires 1.42
Q137: Explain the difference between ΔH and DE.
Q138: Give the temperature and pressure for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents