When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C.If the specific heat of the solution is 4.18 J/(g ∙ °C) ,calculate  for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g) 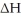 = ?
= ?
A) -431 kJ
B) -3.14 kJ
C) +3.14 kJ
D) +431 kJ
Correct Answer:
Verified
Q18: Calculate the change in internal energy (ΔE)for
Q118: According to the following reaction,how much energy
Q121: Which of the following processes is exothermic?
A)
Q122: Identify a substance that is in its
Q124: Choose the reaction that illustrates ΔH°f for
Q124: Describe the energy changes that occur when
Q127: Identify what a coffee cup calorimeter measures.
A)
Q128: In the presence of excess oxygen,methane gas
Q137: When 10.00 moles of H2(g)reacts with 5.000
Q137: Explain the difference between ΔH and DE.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents