A fixed amount of gas at 25.0°C occupies a volume of 10.0 L when the pressure is 667 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.88 L at a constant temperature of 25.0°C.
A) 846 torr
B) 0.118 torr
C) 5.26 × 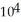 torr
torr
D) 526 torr
E) 1.11 torr
Correct Answer:
Verified
Q3: What pressure (in atm)will 0.44 moles of
Q80: What volume will a balloon occupy at
Q84: What pressure will 2.6 × 1023 molecules
Q86: A fixed amount of gas at 25.0°C
Q89: How many molecules of He are contained
Q90: Calculate the temperature,in K,of 2.20 moles of
Q121: A basketball is inflated to a pressure
Q127: How many molecules of N2 are in
Q134: A gas bottle contains 0.250 mol of
Q136: A 4.00-L flask contains nitrogen gas at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents