Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 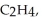 flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain same number of molecules.
Correct Answer:
Verified
Q36: Which of the following samples has the
Q89: Which of the following samples will have
Q100: The density of nitric oxide (NO)gas at
Q110: A 1.44-g sample of an unknown pure
Q112: What volume will 0.780 moles of Xe
Q113: Give the temperature and pressure at STP.
A)
Q118: Given three cylinders containing O2 gas at
Q122: A steel bottle contains argon gas at
Q143: The density of krypton gas at 1.21
Q144: Which of the following would have a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents