A 1.44-g sample of an unknown pure gas occupies a volume of 0.335 L at a pressure of 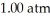 and a temperature of 100.0°C.The unknown gas is
and a temperature of 100.0°C.The unknown gas is
A) argon
B) helium
C) krypton
D) neon
E) xenon
Correct Answer:
Verified
Q36: Which of the following samples has the
Q89: Which of the following samples will have
Q108: Three identical flasks contain three different gases
Q112: What volume will 0.780 moles of Xe
Q113: Give the temperature and pressure at STP.
A)
Q114: What volume will 4.91 × 1022 atoms
Q118: Given three cylinders containing O2 gas at
Q122: A steel bottle contains argon gas at
Q129: A gas occupies 22.4 L at STP
Q143: The density of krypton gas at 1.21
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents