The fluorocarbon C2CL3F3 has a normal boiling point of 47.6°C.The specific heats of C2CL3F3(1) and C2CL3F3(g) are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 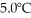 to the gas at 80.0°C is ________ kJ.
to the gas at 80.0°C is ________ kJ.
A) 8.19
B) 1454
C) 30.51
D) 3031
E) 10.36
Correct Answer:
Verified
Q97: Choose the pair of substances that are
Q98: Choose the pair of substances that are
Q99: Identify the compound that has hydrogen bonding.
A)
Q100: Choose the substance with the highest viscosity.
A)
Q101: Assign the appropriate labels to the phase
Q103: Place the following substances in order of
Q104: Choose the substance with the lowest boiling
Q106: The boiling point of water is
A) 0
Q107: Place the following substances in order of
Q123: The heat of vaporization of water at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents