Assign the appropriate labels to the phase diagram shown below. 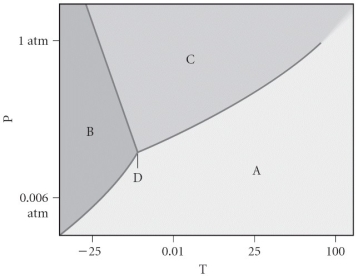
A) A = liquid, B = solid, C = gas, D = critical point
B) A = gas, B = solid, C = liquid, D = triple point
C) A = gas, B = liquid, C = solid, D = critical point
D) A = solid, B = gas, C = liquid, D = triple point
E) A = liquid, B = gas, C = solid, D = critical point
Correct Answer:
Verified
Q96: Place the following compounds in order of
Q97: Choose the pair of substances that are
Q98: Choose the pair of substances that are
Q99: Identify the compound that has hydrogen bonding.
A)
Q100: Choose the substance with the highest viscosity.
A)
Q102: The fluorocarbon C2CL3F3 has a normal boiling
Q103: Place the following substances in order of
Q104: Choose the substance with the lowest boiling
Q106: The boiling point of water is
A) 0
Q123: The heat of vaporization of water at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents