Which of the following represents the equation for a first-order half-life?
A) t 1/2 = 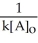
B) t 1/2 = 
C) t 1/2 = 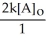
D) t 1/2 = 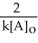
E) t 1/2 = 
Correct Answer:
Verified
Q71: Given the following balanced equation,determine the rate
Q73: Determine the rate law and the value
Q101: The reaction that occurs in a Breathalyzer,a
Q103: The isomerization of methylisonitrile to acetonitrile CH3NC(g)→
Q103: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q105: How many half-lives are required for the
Q106: The rate constant for a second-order reaction
Q162: The decomposition of dinitrogen pentoxide is described
Q170: For a reaction that follows the general
Q172: What is the overall reaction order for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents