The decomposition of 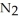
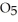 in solution in carbon tetrachloride proceeds via the reaction 2
in solution in carbon tetrachloride proceeds via the reaction 2 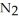
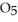 (soln) →
(soln) → 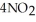 (soln) +
(soln) + 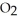 (soln)
(soln)
The reaction is first order and has a rate constant of 4.82 × 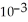
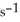 at 64°C.If the reaction is initiated with 0.058 mol in a 1.00-L vessel,how many moles remain after 151 s?
at 64°C.If the reaction is initiated with 0.058 mol in a 1.00-L vessel,how many moles remain after 151 s?
A) 0.055 M
B) 0.060 M
C) 0.028 M
D) 12 M
E) 2.0 × 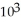 M
M
Correct Answer:
Verified
Q102: For a particular first-order reaction,it takes 24
Q105: How many half-lives are required for the
Q109: Q110: If the concentration of a reactant is Q111: Which of the following represents the equation Q111: How many half-lives are required for the Q113: How many half-lives are required for the Q114: Given the following rate law,how does the Q116: The isomerization of methylisonitrile to acetonitrile CH3NC(g)→ Q117: The rate constant for a zero-order reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents