Which of the following statements is NOT true for weak bases?
A) Weak bases partially hydrolyze water.
B) The conjugate acid is BH+ and its strength is strong.
C) 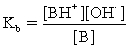
D) 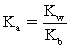
For the conjugate acid.
E) B + H2O ⇋ BH+ + OH−
Correct Answer:
Verified
Q10: A student calculates the pOH for a
Q11: A buffer is prepared by mixing 200.0
Q12: The pH of a weak-acid solution is
Q13: A 0.01 M solution of a weak
Q14: Calculate the pH of 0.01 M
Q15: Calculate the percent dissociation for a 0.01
Q16: There are two types of metal
Q17: The buffer needed for a capillary
Q18: Calculate the pH of a 0.2 M
Q19: One liter of a pH 5.00 propionic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents