Which of the following is NOT true for weak acids and weak bases?
A) Kw = Ka.Kb
B) 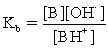
C) 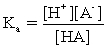
D) pKa = −log Ka
E) pKb = −log Kb
Correct Answer:
Verified
Q2: Calculate the percentage dissociation for a 0.010
Q3: For a 0.0005 M HBr solution,the dissociation
Q4: Which is NOT a property of buffers?
A)Buffers
Q5: The rule of thumb for neglecting the
Q6: Which of the following statements is NOT
Q7: Calculate the pH for a 4.3
Q8: There are seven common strong acids.Which
Q9: The pH of a weak-base solution is
Q10: A student calculates the pOH for a
Q11: A buffer is prepared by mixing 200.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents