A buffer is prepared by dissolving 70 millimoles of solid NaOH in 500.0 mL of solution with a concentration of 50 mM H2A.Which of the following are NOT true for the buffer?
I After reaction there is 30 mmol HA− and 20 mmol A2− in solution.
II pH is calculated using 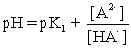 .
.
III After reaction there is 50 mmol HA− and 20 mmol NaOH in solution.
IV pH is calculated using 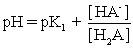 .
.
V pH = 14 - pOH where pOH is calculated from excess OH−.
A) III,IV,and V
B) II,III,IV,and V
C) 1,III,and IV
D) I,II,and V
E) I and II
Correct Answer:
Verified
Q10: Which of the following is TRUE for
Q11: Calculate the pH of a 0.340 M
Q12: For the polyprotic acid H3A,which of the
Q13: Sulfurous acid is a diprotic acid
Q14: Sodium hydroxide is added to a 0.1
Q15: Calculate the pH at the isoelectric point
Q16: Which is NOT true for isoelectric and
Q18: For a weak acid,the
Q19: Calculate the pH to which a malonic
Q20: How many millimoles of NaOH or HCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents