Which statement is incorrect for conditional formation constants,Kf′?
A) Kf′ is pH dependent.
B) Kf′ 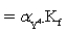
C) Kf′ allows the view of uncomplexed EDTA as being in only one form,Y4−,in equilibrium calculations.
D) As pH drops the value of Kf′ increases.
E) The values of Kf′ must be large to guarantee sharp end points during titrations.
Correct Answer:
Verified
Q10: Calculate pBa when 35.00 mL 0.1 M
Q11: For EDTA titrations the titration reaction is
Q12: Calculate [Mn2+] when 50.00 mL of 0.1000
Q13: Which statement is NOT true?
A)A monodentate ligand
Q14: EDTA is a hexadentate ligand containing four
Q15: Calculate pBa when 50.00 mL 0.1 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents