Multiple Choice
Calculate pBa when 50.00 mL 0.1 M EDTA is added to 50.00 mL of 0.1 M Ba2+.For the buffered pH of 10, 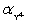 = 0.30.Kf = 7.59 × 107 for BaY2−.
= 0.30.Kf = 7.59 × 107 for BaY2−.
A) 1) 30
B) 7) 88
C) 4) 59
D) 7) 36
E) 4) 33
Correct Answer:
Verified
Related Questions
Q10: Calculate pBa when 35.00 mL 0.1 M
Q11: For EDTA titrations the titration reaction is
Q12: Calculate [Mn2+] when 50.00 mL of 0.1000
Q13: Which statement is NOT true?
A)A monodentate ligand
Q14: EDTA is a hexadentate ligand containing four
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents