Calculate the potential for a redox titration when 50.00 mL of 0.100 M Co3+ is titrated with 35.00 mL of 0.130 M 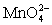 .The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V
.The potential is measured against the Ag-AgCl reference electrode,E = 0.197 V. Co3+ + e- ⇋ Co2+____________________Eo = 1.92 V  + e- ⇋
+ e- ⇋ 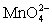 Eo = 0.56 V
Eo = 0.56 V
A) 0) 42 V
B) 0) 62 V
C) 1) 66 V
D) 1) 86 V
E) 1) 24 V
Correct Answer:
Verified
Q2: Potassium permanganate is a strong oxidant commonly
Q3: Sodium thiosulfate is used almost exclusively to
Q4: Potassium permanganate is NOT a primary standard.Why?
I
Q5: For redox titrations,the oxidation state of the
Q6: _ is the titration of iodine produced
Q8: Starch is an indicator for iodine.For iodimetry,starch
Q9: A student is given the assignment to
Q10: Calculate the potential for a redox titration
Q11: Which of the statements below are true
Q12: In addition to redox indicators and starch-iodine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents