A student is given the assignment to determine the mass iron in a brand of women's vitamins.He begins by taking four vitamin tablets,grinding the tablets into a fine powder to undergo the necessary sample preparation to free all iron from the sample matrix and to reduce all iron to the +2 state.The resulting Fe2+ solution is transferred to a 250 mL volumetric flask and diluted to volume with distilled water.A 50 mL aliquot of the sample requires 42.98 mL of 1.00 x 10−3 M 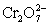 .Calculate the mg Fe per tablet.
.Calculate the mg Fe per tablet. 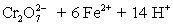 ⇋
⇋ 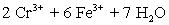
Correct Answer:
Verified
Q4: Potassium permanganate is NOT a primary standard.Why?
I
Q5: For redox titrations,the oxidation state of the
Q6: _ is the titration of iodine produced
Q7: Calculate the potential for a redox titration
Q8: Starch is an indicator for iodine.For iodimetry,starch
Q10: Calculate the potential for a redox titration
Q11: Which of the statements below are true
Q12: In addition to redox indicators and starch-iodine
Q13: Which statements are true for redox indicators?
I.The
Q14: Which are TRUE for the use of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents