The Karl Fischer titration measures the traces of water in samples such as transformer oils,foods,polymers,and so forth.Which of the following is NOT true for the chemistry involved?
I The anode solution contains iodide;an alcohol,ROH;a base,B;sulfur dioxide;and possibly other organic compounds.
II ROH + SO2 + B → BH+ + 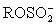 III I2 is generated from I− at the cathode.
III I2 is generated from I− at the cathode.
IV H2O + I2 + 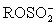 + 2 B →
+ 2 B → 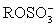 + 2 BH+I-
+ 2 BH+I-
V Two moles of e− corresponds to one mole H2O
A) III only
B) I and III
C) I,III,and V
D) II and IV
E) V only
Correct Answer:
Verified
Q1: Molecules have three ways to reach the
Q3: Which is NOT true of electrogravimetric analysis?
A)Analyte
Q4: For a three-electrode cell the working electrode
Q5: When a potential is applied to a
Q6: Which are true for polarography?
I Polarography is
Q7: The_ is the electrode at which the
Q8: Which statement(s)is(are)defined INCORRECTLY?
I Ohmic potential is the
Q9: An experiment measured the cathodic current
Q10: Which is NOT true of overpotential?
A)Overpotential is
Q11: A technician is tasked with testing the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents