For the Acid-Base Indicator Bromothymol Blue,the Protonated Form,HBB,is Yellow and the Deprotonated
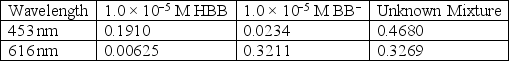
For the acid-base indicator bromothymol blue,the protonated form,HBB,is yellow and the deprotonated form,BB−,is blue.When both forms are present the indicator has a green color,the shade of green depends on the ratio of HBB:BB−.Use the information below to calculate the concentration of HBB and BB− for a green solution of bromothymol blue.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: A student determines the concentration chloride in
Q4: Which is true for the relationship between
Q5: The advantages of flow injection analysis include
Q6: For the method of continuous variation,which is
Q7: _enhances the fluorescence of immunoassays by
Q9: Which is NOT true for a Scatchard
Q10: The stoichiometry for the complex MXn is
Q11: The Stern-Volmer equation relates quencher concentration
Q12: Which is true for spectroscopy of a
Q13: The technique in which a liquid sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents