Consider the following particulate-level representation.The larger spheres represent N and the smaller spheres represent H. 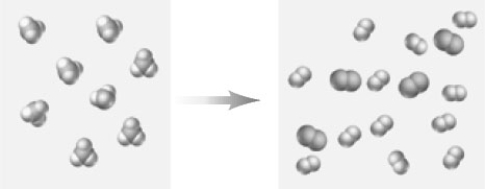 Which of the following correctly characterizes this representation?
Which of the following correctly characterizes this representation?
A) The reactant could be represented as NH3.
B) The products of the reaction are N2 and H2.
C) The equation is "balanced".
D) If the equation for the reaction were written as shown above the coefficient of the nitrogen containing product would be 4.
E) All of these correctly characterize this reaction.
Correct Answer:
Verified
Q2: Balance the equation C8H18(l)+ _ O2(g)→ _
Q3: Which of the following generally indicates the
Q4: Calcium combines with bromine to make calcium
Q5: What is the coefficient for H2 when
Q6: A chemical equation may sometimes be balanced
Q7: 12.09 g of A2 reacts with 96.00
Q8: Consider the hypothetical chemical reaction represented by
Q9: Consider the hypothetical chemical reaction represented by
Q10: What is the coefficient for carbon dioxide
Q11: When atomic phosphorous (P)and oxygen are directly
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents