Consider the following reaction coordinate diagram. 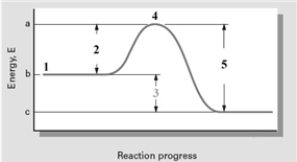 Based on this diagram,which of the following is correct?
Based on this diagram,which of the following is correct?
A) 2 represents ΔE and the reaction is exothermic.
B) 2 represents ΔE and the reaction is endothermic
C) 3 represents ΔE and the reaction is endothermic
D) 3 represents ΔE and the reaction is exothermic
E) 5 represents ΔE and the reaction is exothermic
Correct Answer:
Verified
Q12: What kind of molecular collisions will not
Q13: Consider the particulate level representation of a
Q14: A molecular collision is sufficiently energetic to
Q15: How does a catalyst alter the rate
Q16: Which of the following is the primary
Q18: How does increasing the temperature increase the
Q19: Consider the hypothetical reaction A + 2
Q20: Which of the following statements is/are incorrect?
i.Undissolved
Q21: Choose the correct equilibrium constant expression for
Q22: Choose the correct equilibrium constant expression for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents