Determine the STP volume of sulfur hexafluoride that will be produced from the reaction of 25.0 kg of sulfur in the reaction S( 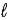 ) + 3 F2(g) → SF6(g) .
) + 3 F2(g) → SF6(g) .
A) 0.0348 kL
B) 34.8 kL
C) 0.0175 kL
D) 17.7 kL
E) 1.75 × 104 kL
Correct Answer:
Verified
Q27: What is the volume of 0.0775 mole
Q28: Consider the reaction CH4(g)+ H2O(g)→ 3 H2(g)+
Q29: What is the molar mass of a
Q30: Calculate the molar volume of nitrogen at
Q31: How many grams of hydrogen chloride are
Q33: What volume of oxygen,measured at STP,can be
Q34: Calculate the molar volume of a gas
Q35: Consider the reaction 2 NH3(g)+ CO2(g)→ H2NCONH2(s)+
Q36: The molar volume of a gas at
Q37: How many liters of hydrogen,measured at 20°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents