ΔH = +572 kJ for the decomposition of water by the reaction 2 H2O( 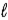 ) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
A) 157 g
B) 315 g
C) 0.0629 g
D) 2.57 × 107 g
E) 5.15 × 107 g
Correct Answer:
Verified
Q20: In the reaction CaCN2 + 3 H2O
Q21: Cu2HgI4 is prepared according to the equation
Q22: Calculate the mass of Na2O that can
Q23: 1.90 × 103 J of heat,in combination
Q24: In the reaction 2 AgI + HgI2
Q26: Consider the following thermochemical equation: CaO(s)+ H2O(
Q27: If the reaction N2 + 3 H2
Q28: The thermochemical equation describing the heat change
Q29: What is the theoretical yield of phosphorus
Q30: When C2H5Cl(g)is burned in oxygen,chlorine gas is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents