Express the equilibrium constant for the following reaction.
2 P(g) + 3 Cl2(g) ⇌ 2 PCl3(g)
A) K = 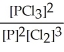
B) K = 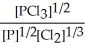
C) K = 
D) K = 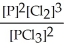
E) K = 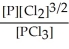
Correct Answer:
Verified
Q4: The equilibrium constant is given for one
Q11: Determine the value of Kc for the
Q14: The equilibrium constant is given for one
Q15: Express the equilibrium constant for the following
Q17: What is △n for the following equation
Q18: What is △n for the following equation
Q20: What is △n for the following equation
Q21: Which of the following statements are true?
A)Dynamic
Q23: For which reaction will Kp = Kc?
A)S(s)+
Q24: Calculate P [NO]eq,if P [NOCl]eq = 0.33
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents