Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Cu(s) ∣ Cu2+(aq,0.0032 M) ∣∣ Cu2+(aq,4.48 M) ∣ Cu(s) 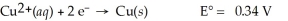
A) 0.00 V
B) 0.093 V
C) 0.34 V
D) 0.186 V
E) 0.052 V
Correct Answer:
Verified
Q43: What mass of silver can be plated
Q62: Match the following.
-Q > K
A)Ecell = E°cell
B)Ecell
Q73: Give the type of battery that most
Q75: Match the following.
-ΔG° < 0
A)Ecell = E°cell
B)Ecell
Q76: Nickel can be plated from aqueous solution
Q107: The gas OF2 can be produced from
Q124: For a galvanic cell that uses the
Q145: What is the difference between a voltaic
Q148: Why are iron nails coated with zinc?
Q151: Based on the following information,
Cl2(g)+ 2 e-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents