In a flask,114.0 g of water is heated using 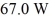
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from 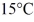
To 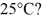
The specific heat of water is 4186 J/kg ∙ K.
A) 71 s
B) 4.1 s
C) 17 s
D) 320,000 s
Correct Answer:
Verified
Q67: A person tries to heat up her
Q68: It is necessary to determine the specific
Q69: If 50 g of lead (of specific
Q70: A container of 114.0 g of water
Q71: A lab assistant pours 330 g of
Q73: A lab assistant drops a 400.0-g piece
Q74: A 200-L electric water heater uses 2.0
Q75: On his honeymoon,James Joule attempted to explore
Q76: A camper is about to drink his
Q77: The water flowing over Niagara Falls drops
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents