Two containers of equal volume each hold samples of the same ideal gas.Container A has twice as many molecules as container B.If the gas pressure is the same in the two containers,the correct statement regarding the absolute temperatures TA and TB in containers A and B,respectively,is
A) TA = TB.
B) TA = 2TB.
C) TA =  TB.
TB.
D) TA = 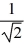 TB.
TB.
E) TA =  TB.
TB.
Correct Answer:
Verified
Q11: The figure shows a graph of the
Q12: How much heat must be removed from
Q13: Oxygen molecules are 16 times more massive
Q14: Two wires are made out of the
Q15: The root-mean-square speed of the molecules of
Q17: A 25-kg piece of equipment can be
Q18: A runner generates 1260 W of thermal
Q19: As one stretches a metal wire,which condition
Q20: A sample of an ideal gas is
Q21: When a sample of water at 0.0°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents